| I. Antifreezes |
| Nature and functions of antifreezes- antifreezes are low-freezing, cooling, and corrosion protecting fluids that lubricates automotive parts and protects them for a longer period of time. |
| 1.1. Low freezing– Depending on the concentration of water in the solution can be reached various freezing temperatures, ranging -10˚C to -60˚C, while the boiling temperature is from + 108˚C to + 197˚C (data are valid for ethylene glycol based products). |
| 1.2. Cooling– In the engine operating mode by combustion of fuel in the engine start process of rapidly increasing temperature, which requires cooling to prevent overheating. Any internal combustion engine (ICE) has a “water jacket” in which flows the antifreeze, or other cooling fluid (water, but it is not recommended), which transfers the heat to the radiator to cool. The process is a continuous circulation during engine operation. |
| 1.3. Heat transfer– Aqueous solution of ethylene glycol is a homogeneous mixture with good heat transfer ability dissipation and uniform heat distribution. |
| 1.4. Corrosion protection– The fluid interaction with metals, rubber parts and turbine equipment and the constant difference in temperature over the time weakens its qualities, thus begins the process of oxidation in the cooling system. So depending on the % MEG (mono ethylene glycol concentrate or ready to use) defined package of antioxidant additives are added to create the so-called “anti-freeze”. |
| 2. Low-freezing fluids without anti-freezing properties. |
| 2.1. NaCl (salt) + H₂O (water) |
| Does not freeze, but is aggressive to metal and rubber seals, no lubricating properties, high acidity. |
| C₃H₈О₃ (glycerin)+ H₂О (water)
Does not freeze. Relatively heavier solution compare with ethyl glycol aantifreeze. Hinder the water pumps work in the system. Oxidizes (sours) due to which may become aggressive for the cooling system. Cooling system corrodes. |
| 2.3. CH₃OH (methanol) + H₂O (water) – Does not freeze, Rapidly evaporates ( + 40˚C). Extremely corrosive for metals and some types of plastics. Extremely flammable. |
| 3. Chemical formulas for antifreeze bases. |
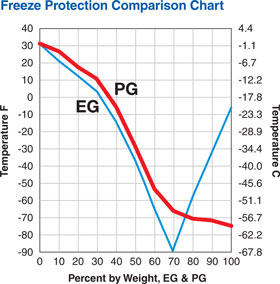
| 3.1. Mono ethylene glycol (MEG) (C₃H₅O₂ – – 1,116 at +20˚C) |
| boiling t˚ + 197˚C; crystallization t˚ -12,9˚C
|
| EG – ethylene glycol |
| PG – propylene glycol |
| a) positive properties (+) |
| Suitable product for the production of antifreeze by adding certain anti-corrosion additive package. |
| See dilution chart depending on the climate and recommended by manufacturer- from -35˚C to -60˚C in the whole system. |
| b) negative properties (-) |
| Toxic, if swallowed, to aquatic animals and humans. |
| 3.2. Propylene glycol (C₃H₈O₂ – 1,04 at +20˚C) |
| Crystallization temperature by 100 % – ( -59˚C) |
| Evaporation temperature – (+ 188˚C) |
| a) positive properties (+) |
| Suitable for production of antifreeze. Used in car racing. .Non-toxic to aquatic organisms, humans and animals. That’s why it is used in the food industry, solar systems and cosmetics. |
| b) negative properties (-) |
| React the less by dilution with water- by 1: 1 ratio the solution reaches freezing temperature of solution of -30˚C (compare with MEG antifreeze (-37˚C) by 1: 1 mixing ratio with H₂O (water). |
| It has the ability to oxidize faster in a working environment. As such requires additional antioxidant additive package. High market price. |
| 3.3. Di-ethylene, tri- and tetra- ethylene glycol (C₄H₁₀O₃ – 1,18 at + 20˚C) |
| Heavier, higher specific weight then ethylene glycol |
| a) positive properties (+) |
| Used mainly for de-icing (de-icing of airplanes), as well as thawing ice on sidewalks, asphalt and cement. |
| b) negative properties (-) |
| Not suitable for the production of antifreezes. Impedes the work of water pumps, very quickly oxidizing. |
| Antioxidants |
| 4.1. Balance of additives- is particularly important that the additive package is properly balanced. Any overdose can have adverse effect on the desired product, while any shortages may worsen the product quality. The types of additives used by antifreeze manufacturing are depending on the product specification. |
| 4.1.1. MEG antifreezes + silicates, phosphates and nitrates additives are recommended for use in older cars. To be replaced every 1 year. |
| 4.1.2. MEG Antifreezes (G11 VW standard) |
| Free of phosphates, nitrates, silicates. Composed of hybrid mineral and organic additives. To be replaced every 3 years (due to higher ingredient quality). |
| 4.1.3. MEG Antifreeze (G12 VW standard)
Composed of organic additives and made according latest technologies. To be replaced every 5 years. |
| Measurement of antifreezes |
| 5.1. Freezing temperature- In order to have an accurate measurement of antifreeze freezing temperature (with exact value) first the antifreeze must be tempered to + 20˚C. See Table 1 |
| Table 1 |
| Ethylene glycol , % |
Initial freezing temperature , t °С |
| 0 |
0 |
| 10 |
-4 |
| 20 |
-7 |
| 30 |
-15 |
| 40 |
-24 |
| 50 |
-38 |
| 60 |
-54 |
| 70 |
-67.8 |
| 80 |
-51.1 |
| 90 |
-35 |
| 100 |
-12 to -17 |
| Note: Data are valid for antifreeze manufactured by MEG. If antifreeze has impurities, such as salt, water glass or glycerin, in order to save costs by producers, this affects the quality of the product (see Table 1) in which measuring devices will show incorrect information. |
| For example: the measuring device will indicate that the antifreeze with the above impurities will not freeze, but at low temperature it will get frozen. |
| The most accurate way of measurement is by using laboratory analysis unit “cryostat” that can achieves a pre-set negative temperature for the tested antifreeze. |
| Tips: When you purchase antifreeze take a look at product concentration and the dilution scheme! Pure concentrate is aggressive and crystallizes at -12,9˚C, so it must be diluted with distilled water. Automakers recommend to be used not more then 40 до 60% antifreeze-concentrate. |
| If you have solution antifreeze- concentrate 67% + 33% water, the solution will theoretically have a freezing point of -72˚C / – 75˚C, but in reality such temperature can not be measured, as even the laboratories” used “cryostat” unit reaches a maximum of -60˚C. As such by purchasing antifreeze with -72˚C; -75˚C; -80˚C you are simply mislead by the producers. |
| When buying antifreeze at -72˚C, in fact this antifreeze contains 33% H₂O (water), so what you purchase is not a concentrate, but antifreeze mixed with water. |
| Scheme 1 |
 |
| This solution graph does not show a straight line (Scheme 1), so if you purchase antifreeze concentrate at -60˚C diluted with distilled water in a 1: 1 ratio the freezing point of this solution will be -18˚C / -20˚C, and when using concentrate with 1: 1 ratio the obtained temperature (according to the standard) is -36˚C to -40˚C (e.g.: if using LONG LIFE VEKO concentrate antifreeze) |
| It is important when you purchase antifreeze to look at packaging for its temperature of crystallization or freezing and the measure of the content. If the label shows kilograms and the price is per kilograms, note that this is not as one liter because: |
| 1 kg. = 0,892 l. |
| 3 kg. = 2,680 l. |
| 4 kg. = 3,571 l. |
| 5 kg. = 4,464 l. |
| According to the law of the Republic of Bulgaria and according to the requirements of EU legislation, all liquids must be packed and labeled in L (liters) |
| II. Winter windscreen fluids |
| Application: to clean, protect, thawed, prevent from freezing, to add shine and flavor of automotive glass, etc. |
| Ingredients: alcohol composition, water + (surface active agents), a set of cleaning, flavorings and coloring additives. |
| 2.1. Methanol based fluids– Methyl alcohol based (CH₃OH). Toxic and poisonous, so by ingestion of even small amounts you must be consulted by medical practitioner. Even small doses (50 ml) can be poisonous for human body. |
| Used only for industrial purposes. Produced by distillation of wood pulp or synthesis of natural gas (methane). |
| Methanol has no specific smell, color and is burning without flame. Suitable for washing fluids as having low freezing temperature (as concentrate). It is also cheaper than ethanol. |
| 2.2. Ethanol based fluids– Based on ethyl alcohol (C₂H₅OH), produced by the distillation of sugar beet, barley, corn, sugar cane and many more. Characterized by a sharp flavor. To avoid excise duty payments some producers are mixing it with ethylmethylketone and isopropanol (poisonous and toxic if swallowed). This solution has even more acute and irritating odor that can not be completely neutralized, even with by adding of larger package of flavors. Blue colored. |
| These liquids are not as poisonous as methanol, but ethanol is more expensive and has lower freezing temperature /concentration. Ethanol also has greater volatility than methanol. |
| 2.3 Isopropanol based fluids – Based on synthesized isotope of ethanol. Less toxic than methanol, but with lower characteristic odor and a lower freezing temperature/concentration than ethanol. This fluid has a higher market price of ethanol and can be aggressive to some plastic parts of cars equipped with headlight with washing devices.. |
| Used mainly by industrial production of paint thinners and varnishes. |